Metal Deactivators and UV-Light Absorbers
Metals can accelerate thermal oxidation of polyolefins and related plastics such as EPDM. To prevent metal catalyzed degradation, it is often neccessary to combine an antioxidant with an metal deactivator if the former does not contain a moity with radical scavenging properties.
The function of a metal deactivator or metal-deactivating agent (MDA) is to form an inactive complex with the catalytically active metal ion. Most chelating agents are well suited for this purpose because they form thermally stable metal complexes. The general feature of chelating agents is their polyfunctionality. They contain several ligand atoms such as N, O, S, P often in combination with functional groups such as hydroxyl, carbonyl, or carbamide. The different substituents will affect the properties, like polarity, volatility, miscibility, and UV absortion spectrum. Common metal deactivators include salicylidene propylenediamine (1), oxyalyl bis(benzylidene)hydrazine, citric acid, ethylene-diaminetetraacetic acid (2) and its salts, mercaptobenzothiazoles, mercaptobenzimidazoles and many thiadiazole and triazole derivatives to name only a few.
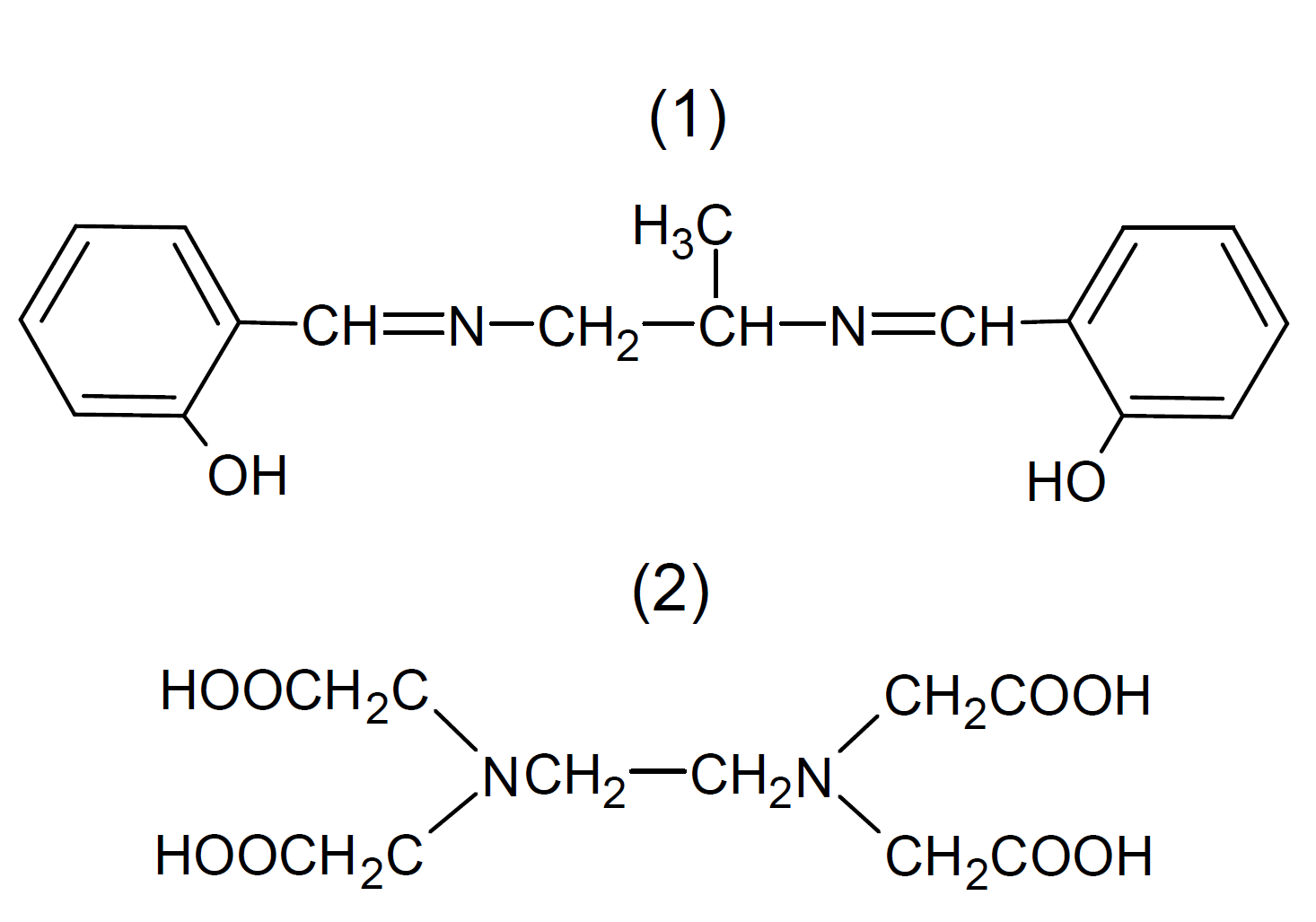
Many metal deactivators also function as UV-Absorbers by competitive absorption of destructive UV radiation. These compounds generally absorb UV light much more strongly than the polymers that they protect. The excited states of these compounds relax to the ground state very rapidly and efficiently without producing any radiation, which imparts high UV stability.
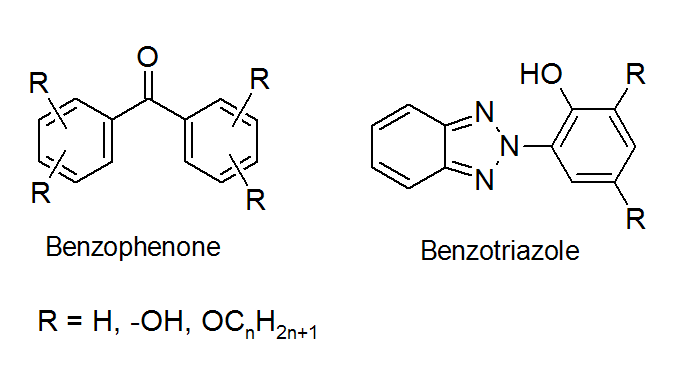
UV-Absorbers are usually only effective in thick and unpigmented/unfilled plastic products, especially when combined with HALS. They are usually not recommended for thin plastic films (below 100 micron) because a certain thickness is required to achieve sufficient UV-absorption.
Another important class of light stabilizers are nickel quenchers. These compounds "quench" the excited states of carbonyl groups, that form during photo oxidation and through decomposition of hydroperoxides. However, these compounds are not widely used because they contain heavy metal and cause discoloration.